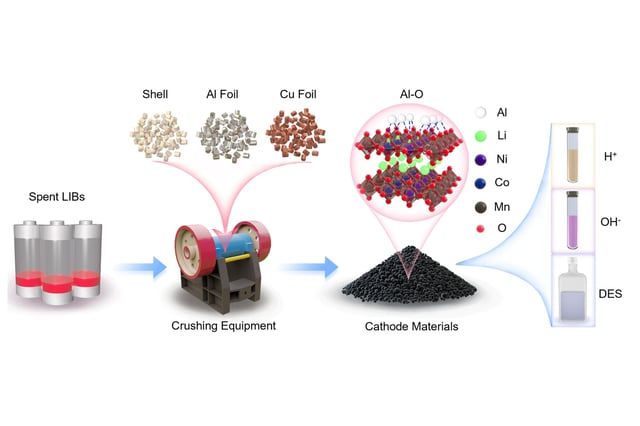
Overview
- Mechanical disassembly of spent lithium-ion batteries embeds residual aluminum foil into nickel–cobalt–manganese cathode crystals through frictional contact.
- Aluminum atoms selectively replace cobalt and form ultra-stable aluminum–oxygen bonds that anchor lattice oxygen and immobilize nickel, cobalt and manganese.
- The effect of aluminum contamination on metal leachability varies by solvent, slowing release in formic acid, accelerating it in ammonia and producing mixed results in deep eutectic solvents.
- Leveraging graphite from anode materials to activate carbon–oxygen bonds lowers the thermal decomposition temperature of cathodes, enabling efficient recovery of lithium carbonate and transition metal oxides.
- The study urges battery recyclers to revise mechanical disassembly methods and solvent protocols to enhance metal recovery yields and energy efficiency in closed-loop systems.