Overview
- An international Delphi consensus of 89 experts from 17 countries led by McGill, Imperial College London and the University of Exeter formulated the ReSPCT guidelines.
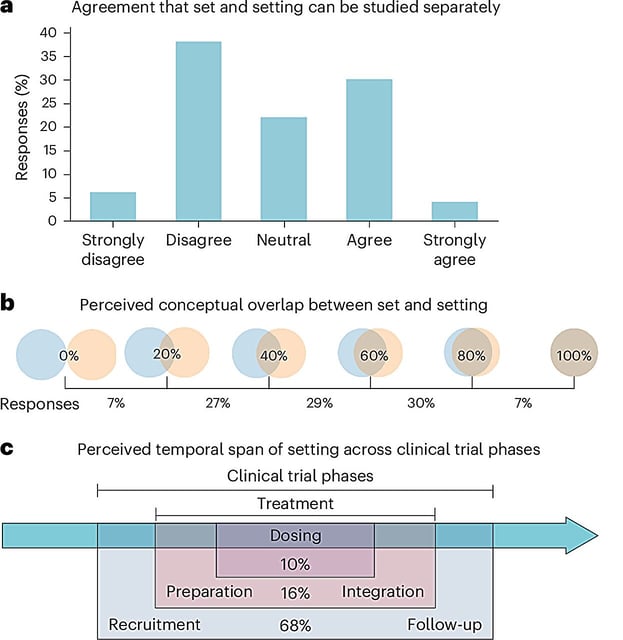
- The 30-item checklist requires systematic reporting of psychosocial variables—including participant mindset, physical setting and therapist-patient interactions—to capture core 'set and setting' factors.
- Published in Nature Medicine, the guidelines represent the first global agreement on integrating contextual elements into the design and reporting of psychedelic studies.
- The framework aims to address inconsistent data that contributed to the U.S. FDA’s recent rejection of MDMA-assisted therapy for PTSD by improving trial comparability.
- An intensive three-day workshop in October 2025 will guide researchers on implementing the ReSPCT standards in ongoing and future psychedelic clinical trials.