Overview
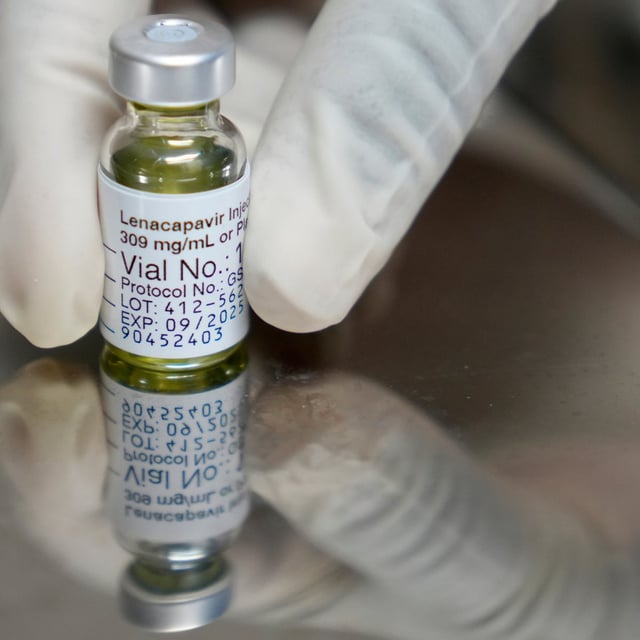
- Phase 3 trials showed lenacapavir’s twice-yearly injection achieved 100% efficacy in preventing HIV infection in cisgender women.
- U.S. regulatory approval is expected on June 19, paving the way for the first long-acting preventive HIV injection in America.
- Gilead partnered with six generic manufacturers to produce low-cost versions of lenacapavir for 120 designated low-income countries.
- The company pledged to supply no-profit doses of lenacapavir to about 2 million people before generic versions become available.
- Advocates warn that an expected U.S. list price near $25,000 per year and licensing exclusions for key middle-income nations could hinder broader access.