Overview
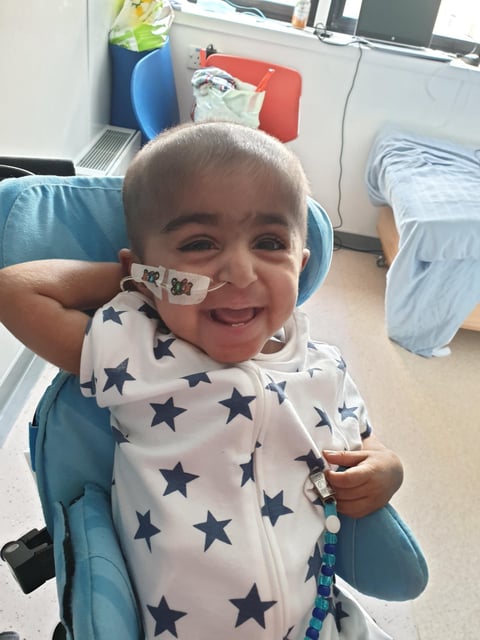
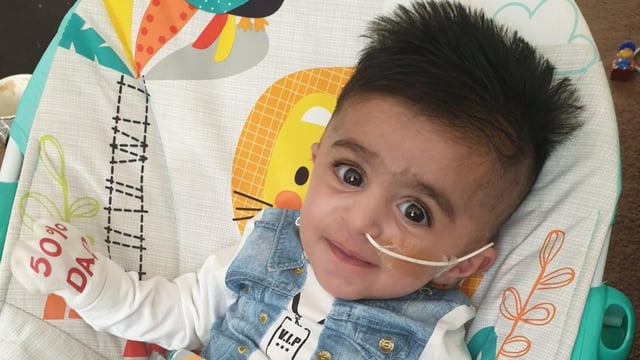
- Nine children with severe leukocyte adhesion deficiency-I (LAD-1) achieved immune restoration following lentiviral gene therapy, avoiding the life-threatening prognosis of the disease.
- The therapy reprograms patients' stem cells to produce the missing CD18 protein, enabling their immune systems to fight infections effectively.
- Two-year follow-up data published in the New England Journal of Medicine confirmed all patients survived, with durable CD18 expression levels reaching up to 82% in some cases.
- The trial, sponsored by Rocket Pharmaceuticals, showed no therapy-related deaths or severe adverse events, with participants experiencing fewer infections and improved wound healing.
- The U.S. FDA is currently reviewing the therapy's Biologics License Application, raising hopes for its wider availability and potential application to other genetic and degenerative conditions.