Overview
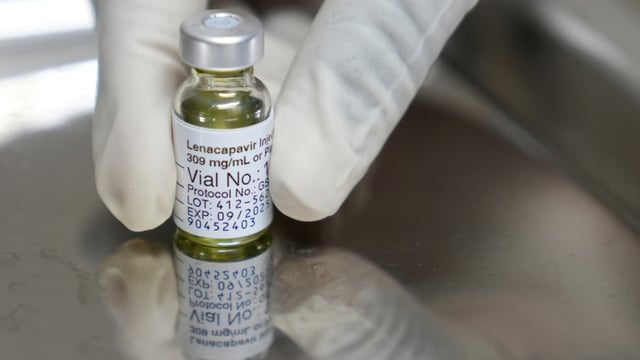
- The U.S. FDA last month approved Gilead Sciences’ twice-yearly injectable lenacapavir for HIV prevention after trials showed near-perfect efficacy in adults and adolescents.
- Gilead and the Global Fund have finalized an at-cost supply agreement to deliver the drug to 2 million people in low-income countries by 2028, with first doses expected in sub-Saharan Africa before year-end.
- Royalty-free licenses with six generic manufacturers will enable low-cost production in 120 low- and middle-income countries, but generic supplies are not projected until around 2027.
- Cuts to PEPFAR and USAID funding under the U.S. administration have eroded testing, counseling and distribution networks, leading to the loss of 8,000 HIV health-worker jobs in South Africa.
- Public health models project that reduced prevention funding could result in at least 70,000 additional HIV infections and 5,000 more deaths over the next five years.