Overview
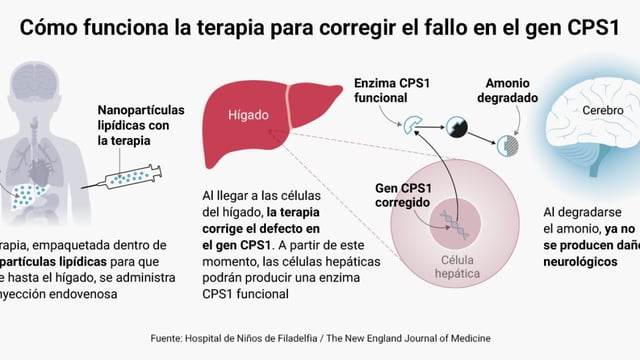
- KJ, a U.S. infant born with CPS1 deficiency, is the first patient successfully treated with a bespoke CRISPR-based gene-editing therapy.
- The therapy restored the liver's ability to produce the CPS1 enzyme, stabilizing KJ's metabolism and improving protein tolerance.
- Developed in just six months, the treatment received FDA approval within one week after rigorous preclinical testing in mice and macaques.
- Results published in The New England Journal of Medicine and presented at the ASGCT conference highlight the therapy's potential for other rare genetic conditions.
- While KJ is growing well and showing improved health, extended monitoring will assess long-term safety and neurological outcomes.