Overview
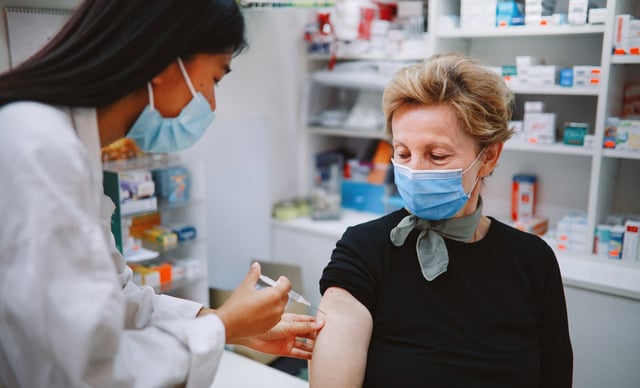
- The FDA has approved the first-ever vaccine for respiratory syncytial virus, called Arexvy, developed by GSK for adults ages 60 and older.
- Arexvy reduces the risk of developing RSV-associated lower respiratory tract disease by 83% and severe RSV-associated lower respiratory tract disease by 94% in older adults.
- RSV is a common virus that causes infections in the lungs and breathing passages and can lead to life-threatening illness in vulnerable populations like young children and older adults.
- The vaccine is expected to be available ahead of the fall RSV season for at-risk seniors in the U.S., pending CDC recommendation.
- Other companies like Pfizer and Moderna are also developing RSV vaccines and treatments currently under regulatory review.