Overview
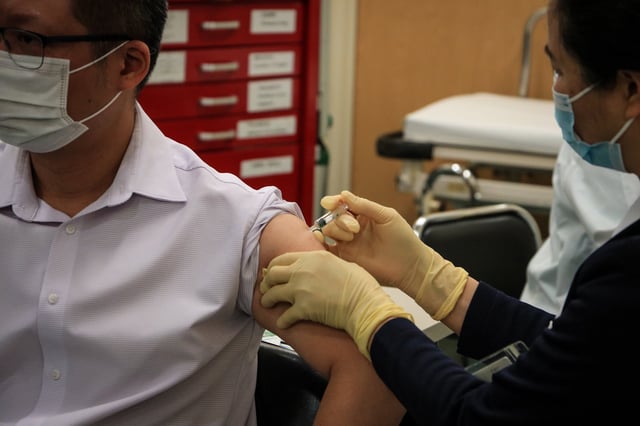
- ACIP ended the blanket recommendation for COVID-19 vaccination, urging shared decision-making with a provider and requiring consults for adults 65 and older, while a proposal to mandate prescriptions narrowly failed.
- The panel emphasized that benefits are strongest for high‑risk groups, a shift that could complicate pharmacy access that delivered most shots last season.
- For children under 4, the committee recommended separate measles‑mumps‑rubella and varicella doses instead of the combined MMRV shot, reversing an earlier vote to align guidance across the Vaccines for Children program if adopted by CDC leadership.
- A vote to change the hepatitis B birth‑dose schedule was tabled, leaving the universal newborn dose unchanged for now as HHS urged universal hepatitis B testing during pregnancy.
- States began issuing their own guidance, with an Illinois advisory panel voting to recommend updated COVID-19 shots for all adults and many children pending state approval.