Overview
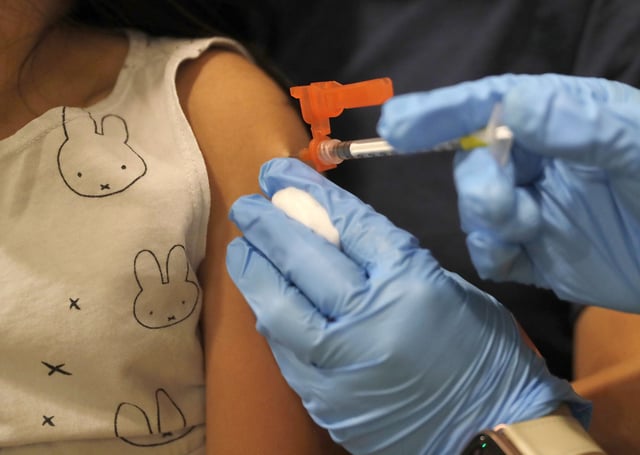
- The FDA has informed Pfizer it may not renew its emergency use authorization for the COVID-19 vaccine in children 6 months to 4 years, and Pfizer says discussions with the agency focus on next-season planning rather than safety or efficacy concerns.
- Allowing the EUA to lapse would leave healthy under-5s without any officially authorized vaccine option, since Moderna’s fully approved shot is restricted to high-risk children and Novavax is not cleared for those under 12.
- In May, HHS Secretary Robert F. Kennedy Jr. removed routine COVID-19 vaccine recommendations for healthy children and pregnant women, prompting the CDC to adopt shared clinical decision-making and the FDA to demand more rigorous trials for low-risk pediatric groups.
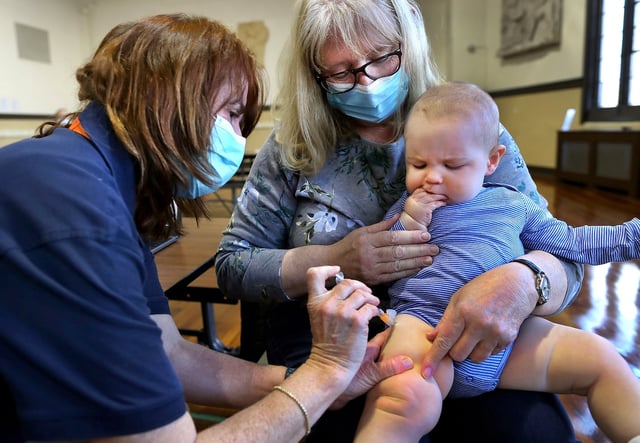
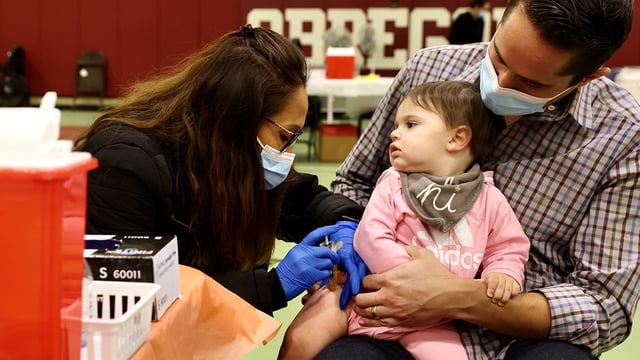
- CDC data last season recorded 48 COVID-19 hospitalizations per 100,000 children under 5—higher than in any other age cohort—leading experts to warn that narrowed access could expose vulnerable infants during the fall respiratory season.
- Clinicians and health departments are gearing up for patchwork off-label use amid unclear insurer obligations, uneven state ordering and potential supply shortages as the next virus season approaches.