Overview
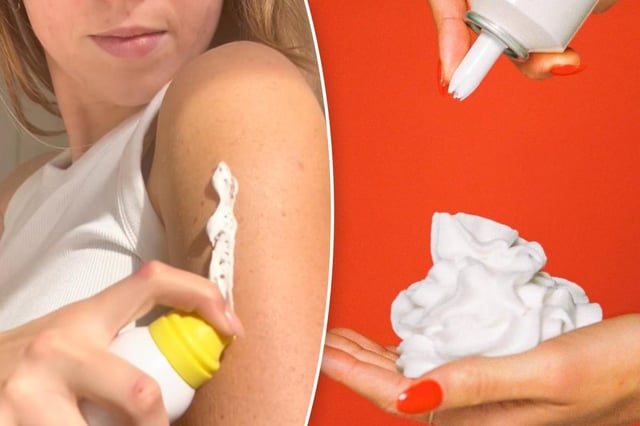
- On August 6 the FDA sent warning letters to five sunscreen brands, including Supergoop! and Vacation, for marketing mousse, whipped and foam formulas that lack approval under existing OTC drug rules.
- Under current U.S. regulations, sunscreens are limited to forms like lotion, cream, spray or stick and require new drug applications for unrecognized formats like mousse.
- The FDA specifically cautioned that Vacation’s whipped-can packaging could mislead consumers and raise the risk of accidental ingestion, particularly among children.
- While Kalani Sunwear has already pulled its mousse product from U.S. sales to comply, other brands say they are engaging with the agency and stress the letters do not question their products’ safety or efficacy.
- Firms now have 15 days to detail corrective measures or dispute the FDA’s classification and industry stakeholders continue to call for regulatory modernization to accommodate innovative sunscreen formats.