Overview
- Counterfeit versions of the popular diabetes drug Ozempic have infiltrated the U.S. drug supply chain, with the FDA seizing thousands of units.
- The FDA and Novo Nordisk, the maker of Ozempic, are currently testing the seized counterfeit drugs to determine their identity, quality, and safety.
- Five illnesses have been linked to the counterfeit Ozempic, but none have been serious. The symptoms were similar to common side effects of Ozempic, like nausea and stomach pain.
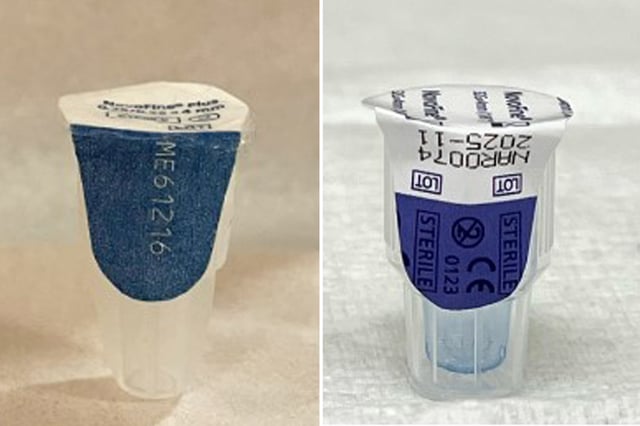
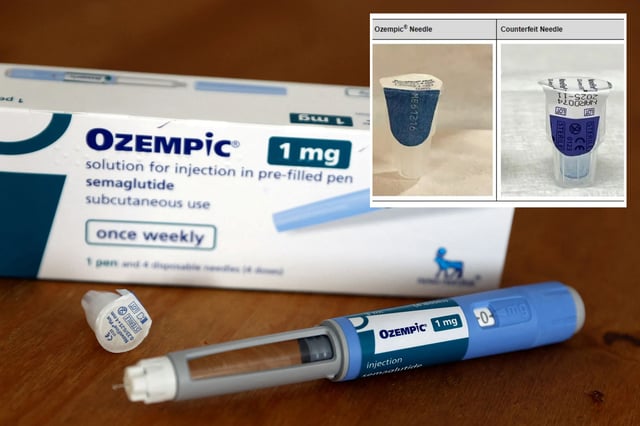
- The counterfeit Ozempic products come with counterfeit needles, pen labels, cartons, fact sheets, and needles, posing an infection risk to consumers.
- The FDA is advising retail pharmacies to buy authentic Ozempic only through authorized distributors and for patients to get it only through state-licensed pharmacies.