Overview
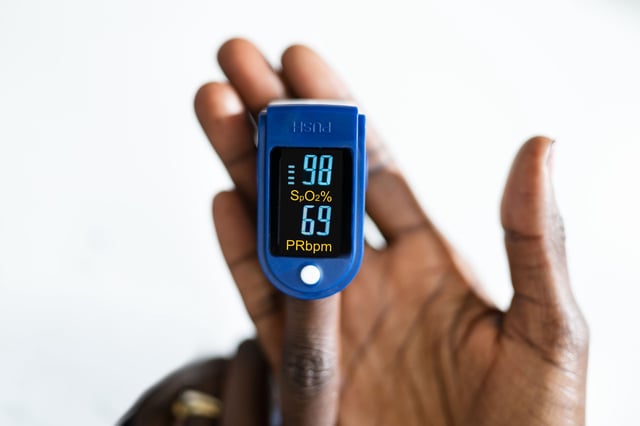
- The FDA has released draft recommendations requiring pulse oximeter manufacturers to conduct more inclusive studies to ensure device accuracy across different skin tones.
- The proposal highlights concerns that darker skin pigmentation may affect the accuracy of oxygen level readings, potentially delaying treatment for patients of color.
- Key recommendations include enrolling at least 150 participants of varying skin tones, ensuring at least 25% of participants have darker skin, and using two methods to assess skin pigmentation in clinical trials.
- The guidance applies only to professional pulse oximeters used in medical settings and excludes over-the-counter devices categorized as general wellness products.
- The draft is open for public comment over the next 60 days before the FDA finalizes the guidelines.