Overview
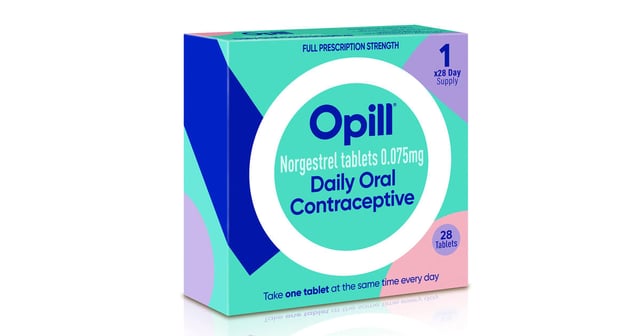
- A panel of independent medical experts for the FDA voted unanimously in favor of allowing the hormonal birth control pill Opill to be sold over the counter.
- HRA Pharma conducted a clinical trial with roughly 900 participants who reported taking the pill, called Opill, correctly more than 90 percent of the time.
- Advocates argue that over-the-counter birth control would benefit people of color and those living in rural, underserved communities who face barriers to accessing healthcare.
- Major medical groups are backing the request, but some groups are opposed, citing safety concerns and potential harm to women's health.
- Making the medication available over the counter should increase access and reduce unintended pregnancies.