Overview
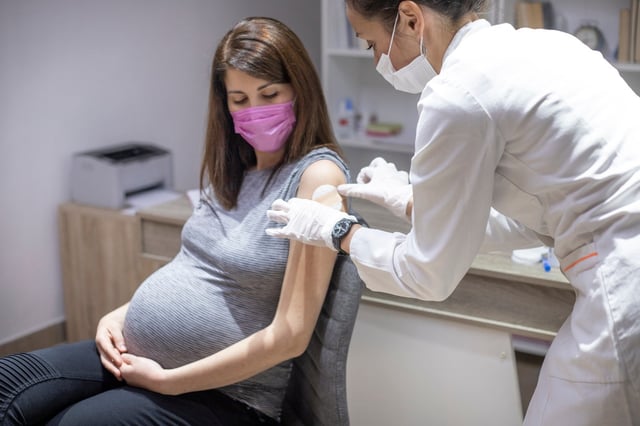
- If approved, the vaccine would be administered during pregnancy to protect infants from RSV, a common respiratory virus and leading cause of hospitalization in babies under 6 months.
- In clinical trials, the vaccine was 82% effective at preventing severe RSV in infants during the first 3 months of life and 69% effective during the first 6 months.
- Some panel members expressed concerns about a slightly higher rate of premature births in the vaccinated group but agreed the data supported the vaccine's safety.
- The vaccine is made by Pfizer and would be the first approved to guard against RSV by vaccinating pregnant women.
- The FDA is expected to make a final decision on approval by late August.