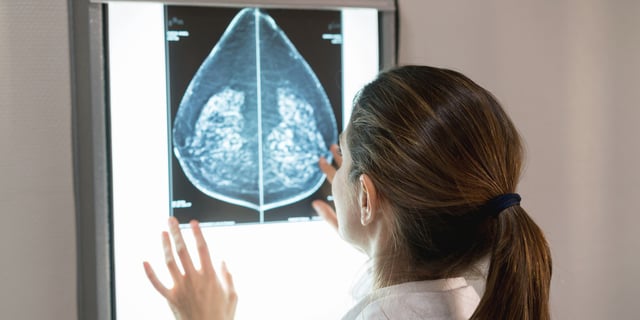
Overview
- New FDA regulations require mammography facilities to inform women about breast density starting September 10, 2024.
- Dense breast tissue can obscure cancer detection on mammograms and increases breast cancer risk.
- Approximately half of women over 40 in the U.S. have dense breasts, impacting their cancer screening process.
- Women with dense breasts may consider additional imaging tests like ultrasound or MRI for better detection.
- The regulation aims to standardize breast density notifications nationwide and improve patient-provider discussions on cancer risk.