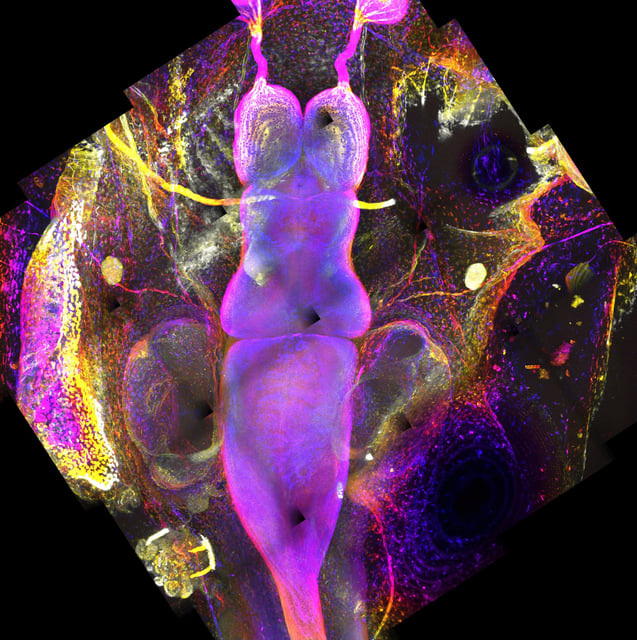
Overview
- Researchers used a CRISPR-engineered Xenopus laevis tadpole model and the nemoCAD pipeline to predict vorinostat as the top repurposing candidate for Rett syndrome.
- In both tadpole and mouse preclinical tests, vorinostat outperformed trofinetide by reversing seizures, motor and gastrointestinal symptoms even after full disease onset.
- Gene network and molecular analyses showed vorinostat restores dysregulated acetylation of histones and α-tubulin across multiple organs, indicating targets beyond traditional HDAC inhibition.
- The FDA’s Orphan Drug Designation for RVL-001—Unravel Biosciences’ proprietary vorinostat formulation—provides regulatory incentives to accelerate development for this rare disorder.
- The planned proof-of-concept n-of-1 trial in Colombia will assess the safety and efficacy of RVL-001 in fifteen girls with Rett syndrome, marking its first human test.