Overview
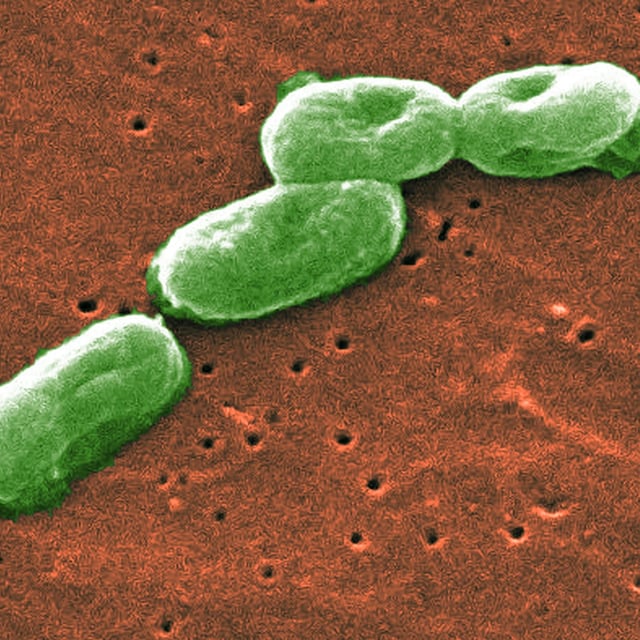
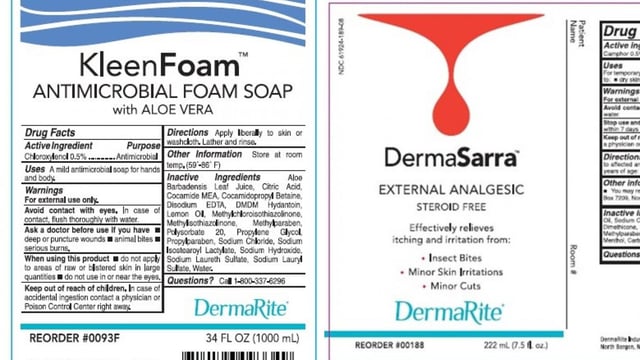
- DermaRite Industries voluntarily recalled specific lots of DermaKleen, DermaSarra, KleenFoam and PeriGiene on August 8 after tests detected Burkholderia cepacia complex in the products
- The FDA published a recall notice on August 9 listing affected lot numbers, expiration dates, reorder codes and product labels for nationwide and Puerto Rico distribution
- DermaRite has emailed distributors and customers with instructions to examine inventory and destroy all impacted products under hazardous‐waste or institutional disposal procedures
- The company reports no confirmed adverse events to date, while the CDC and FDA emphasize the elevated sepsis risk for immunocompromised individuals if exposed to the bacterium
- Investigations into the contamination source remain open and the FDA’s MedWatch program continues to accept reports of any potential adverse reactions