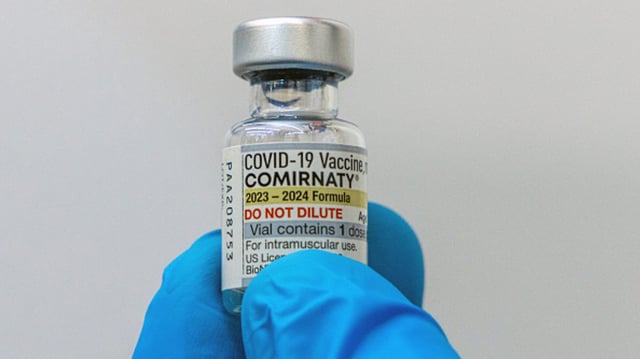
Overview
- The FDA approved Pfizer’s updated COVID-19 shot for the coming season.
- Use is unrestricted for adults 65 and older, while younger adults and children qualify only with a high-risk condition such as asthma or obesity.
- Doses begin shipping immediately, with access contingent on decisions by federal health advisers, private insurers, pharmacies and state authorities.
- The American Academy of Pediatrics objected to the limits, warning they could block families seeking vaccination for children.
- The policy shift reflects skepticism from Health Secretary Robert F. Kennedy Jr. and FDA Commissioner Marty Makary, as local data show cases rising and a 'stratus' strain circulating in parts of the West and South.