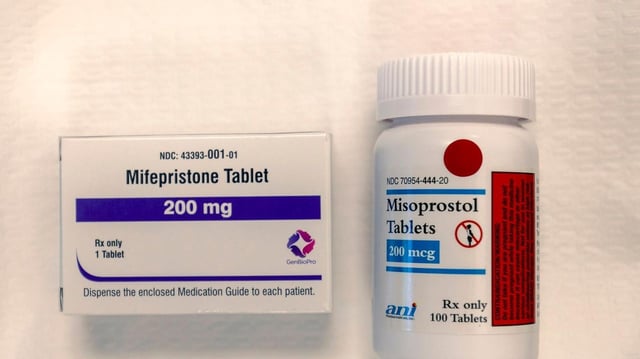
Overview
- The FDA approved Evita Solutions’ generic mifepristone in a Sept. 30 letter issued quietly before a possible government shutdown, expanding U.S. manufacturers to three.
- White House press secretary Karoline Leavitt said the action is not an endorsement and that the agency must approve a generic that proves equivalence to the brand-name drug.
- Sen. Josh Hawley demanded details on Commissioner Marty Makary’s role and warned the timing could make planned safety revisions “toothless and irrelevant.”
- A bloc of House conservatives urged HHS Secretary Robert F. Kennedy Jr. to fire FDA division leaders tied to the approval, while activist groups pressed for reversal and market removal.
- Under current rules, mifepristone can be dispensed via pharmacies and by mail, a key access point that anti-abortion groups want the FDA to tighten as the review continues.