Overview
- The FDA approved MT-125 to enter first-in-human trials after mouse studies demonstrated its ability to overcome glioblastoma resistance to radiation and chemotherapy.
- MT-125 targets non-muscle myosin II to sensitize previously resistant tumor cells to radiation and kinase inhibitors while blocking their invasive behavior.
- Mechanistic research showed the inhibitor disrupts mitochondrial fission, causing reactive oxygen species buildup, DNA damage and ferroptosis in glioblastoma cells.
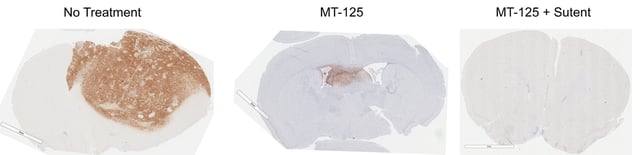
- In preclinical mouse models, MT-125 exhibited strong brain penetrance, minimal toxicity and durable tumor remissions when combined with kinase inhibitors such as sunitinib.
- Myosin Therapeutics licensed MT-125 for imminent clinical development and is also advancing a related compound, MT-110, toward trials for methamphetamine use disorder.