Overview
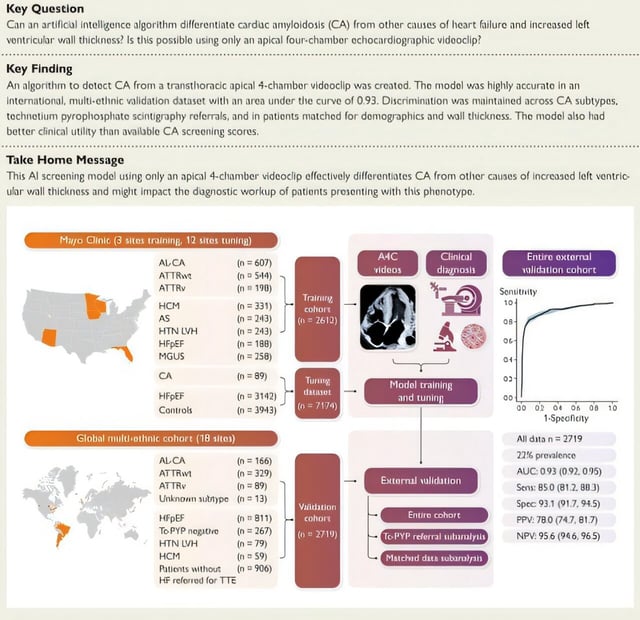
- Developed by Mayo Clinic and Ultromics the algorithm analyzes a single apical four-chamber echocardiogram clip to flag amyloid protein buildup in the heart.
- In a multiethnic study published in the European Heart Journal the tool demonstrated 85% sensitivity and 93% specificity across major amyloidosis subtypes.
- It outperformed standard clinical scoring and echo-based methods to inform decisions on confirmatory imaging or biopsy.
- Clinicians at several U.S. centers have integrated the FDA-cleared system into routine echocardiography workflows for faster patient referral.
- Early identification enables prompt initiation of new therapies that can slow or halt disease progression when started in initial stages.