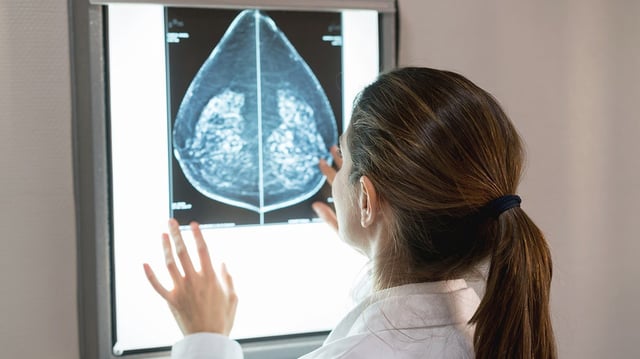
Overview
- The FDA granted De Novo authorization to CLAIRITY BREAST on June 3, 2025, marking the first regulatory clearance for an AI-based breast cancer risk prognostic platform.
- CLAIRITY BREAST uses machine learning to detect subtle imaging features in standard mammograms that correlate with an individual’s five-year cancer risk.
- Risk scores are delivered through existing clinical infrastructures, enabling healthcare providers to tailor screening intervals and consider supplemental methods, such as MRI.
- Traditional models often miss risk factors in women without family history and underrepresent diverse racial and ethnic groups—gaps CLAIRITY BREAST aims to address.
- Clairity, Inc. plans to roll out the platform across leading U.S. health systems through 2025 to advance early detection and reduce late-stage diagnoses.