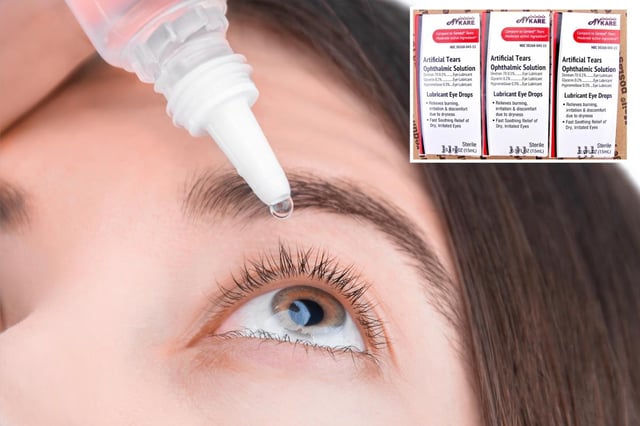
Overview
- The recall affects five eye care products, including artificial tears and lubricant solutions, shipped between May 2023 and April 2025.
- FDA identified deviations from Current Good Manufacturing Practices (cGMP) and a lack of sterility assurance during a routine audit.
- The recall has been classified as Class II, indicating moderate risk of temporary or reversible adverse health consequences.
- Consumers are urged to discontinue use of the products immediately and return them for a full refund, including shipping costs.
- AvKare stated that while no health issues have been reported, patient safety risks cannot be ruled out due to quality concerns.