Overview
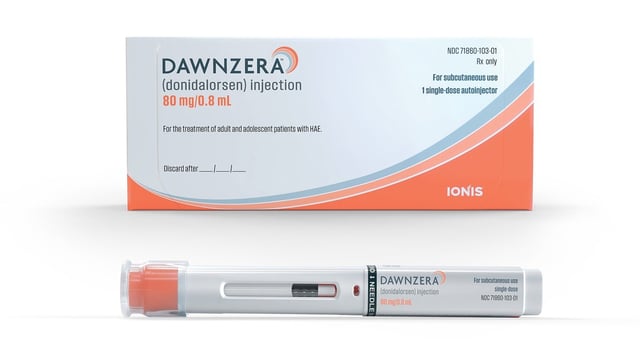
- Ionis’ therapy is cleared for prophylaxis in patients aged 12 years and older.
- The approved presentation is an 80 mg subcutaneous autoinjector administered every four or eight weeks.
- In the Phase III OASIS-HAE trial, monthly attacks fell 81% with four-week dosing versus placebo, with a 55% reduction observed using every-other-month dosing.
- Trial reports noted common side effects including injection-site reactions, urinary and upper respiratory tract infections, and abdominal discomfort.
- The launch enters a crowded HAE market that added Ekterly and Andembry this year, while Ionis plans to market Dawnzera itself and previously said it would disclose pricing on a company call.