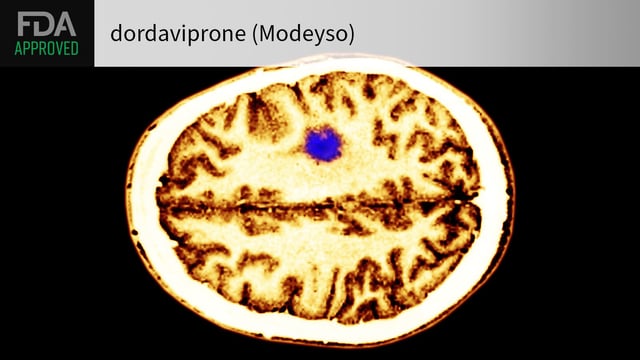
Overview
- Dordaviprone (Modeyso) is authorized for patients aged one year and older with recurrent H3 K27M-mutant diffuse midline glioma following prior therapy
- The approval represents the first systemic treatment option for this aggressive grade IV tumor that traditionally relied on radiation and palliative care
- Regulatory clearance was granted on the basis of pooled data from five open-label studies showing a 22% overall response rate and a median response duration of 10.3 months
- Prescribing information carries warnings for hypersensitivity reactions, QTc interval prolongation and embryo-fetal toxicity
- Jazz Pharmaceuticals anticipates a late-summer commercial launch in the United States with marketing contingent on confirmatory phase III ACTION trial outcomes