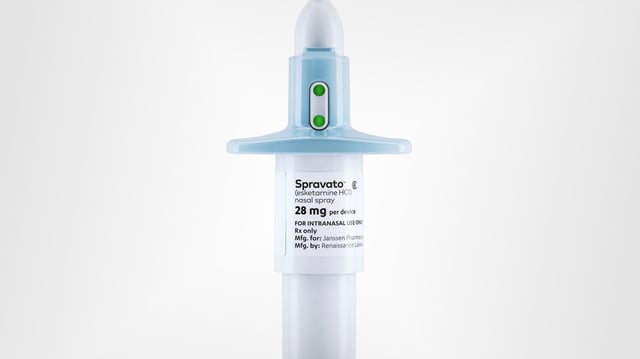
Overview
- Spravato, a nasal spray developed by Johnson & Johnson, is the first FDA-approved standalone therapy for treatment-resistant depression.
- The drug, derived from esketamine, targets the neurotransmitter glutamate and can reduce depressive symptoms in as little as 24 hours.
- Previously approved in 2019 as an add-on therapy, Spravato's expanded approval is based on a study showing significant improvements compared to a placebo.
- Due to potential side effects, including sedation and dissociation, the treatment is only available through certified centers under strict medical supervision.
- This approval provides a new option for the estimated one-third of U.S. adults with major depressive disorder who do not respond to traditional antidepressants.