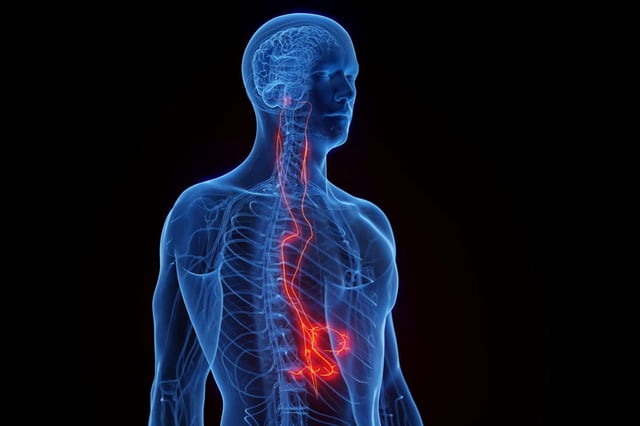
Overview
- The FDA has cleared the SetPoint System for moderate-to-severe rheumatoid arthritis in patients who have not responded to or cannot tolerate standard DMARD therapies.
- The implant is placed in the neck and delivers a 60-second, low-voltage pulse to the vagus nerve each day to trigger anti-inflammatory neural pathways.
- Approval was based on a randomized, double-blind, sham-controlled trial of 242 patients in which treated participants achieved higher ACR20 response rates and 75% were free of biologic or targeted synthetic DMARDs at one year.
- SetPoint Medical will begin a phased U.S. launch in select cities later in 2025, followed by a nationwide expansion planned for early 2026 under the FDA’s Breakthrough Devices program.
- The company is advancing clinical trials to evaluate vagus nerve stimulation for multiple sclerosis and Crohn’s disease