Overview
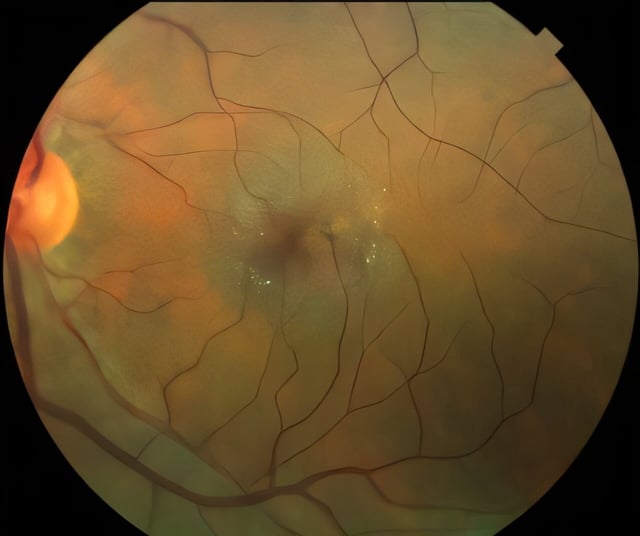
- ENCELTO (revakinagene taroretcel-lwey) secured FDA approval on March 6, 2025 as the first treatment for macular telangiectasia type 2, a disease that progressively impairs central vision.
- The implant uses genetically engineered retinal pigment epithelial cells to secrete ciliary neurotrophic factor, supporting photoreceptor survival and delaying degeneration.
- Two phase 3 studies demonstrated slowed loss of light-sensing retinal cells over 24 months, and explanted devices maintained therapeutic CNTF levels after 14.5 years.
- Development stemmed from a collaboration between Scripps Research, the Lowy Medical Research Institute and Neurotech Pharmaceuticals, driven by a Lowy family member’s MacTel diagnosis.
- Investigators are now evaluating ENCELTO’s potential in treating other neurovascular conditions such as glaucoma and age-related macular degeneration.