Overview
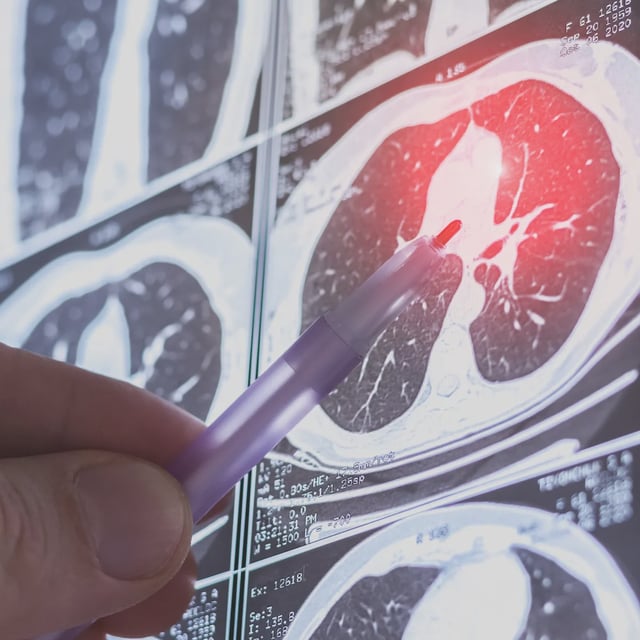
- The approval covers Brinsupri (brensocatib) 10 mg and 25 mg once-daily tablets for adults and patients 12 years and older with non-cystic fibrosis bronchiectasis.
- Phase 3 ASPEN results showed a 21.1% reduction in annual exacerbations at 10 mg and 19.4% at 25 mg versus placebo, with both doses delaying first flare-ups and boosting exacerbation-free rates.
- Phase 2 WILLOW safety data aligned with ASPEN findings except for a higher incidence of gingival and periodontal adverse reactions, which will inform post-marketing monitoring.
- Insmed’s stock jumped more than 6% on the approval news and Wall Street forecasts peak annual sales of up to $6 billion at a list price of about $88,000 per patient.
- The company has secured EMA and MHRA filings, plans a 2025 submission in Japan and is targeting commercial launches in 2026 pending foreign approvals.