Overview
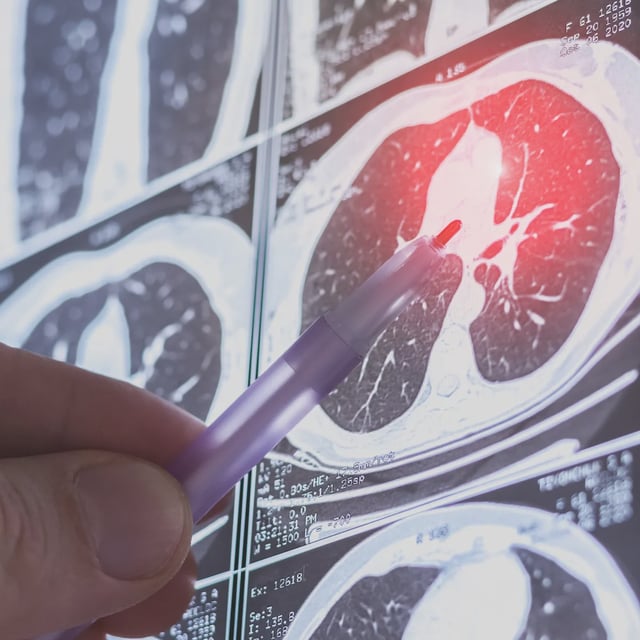
- Brensocatib, marketed as Brinsupri, is approved for adults and children 12 years and older based on WILLOW and ASPEN trials that showed 19–21% reductions in pulmonary exacerbation rates and extended time to first flare-up.
- The drug is the first on-label dipeptidyl peptidase-1 inhibitor for non-CF bronchiectasis, targeting neutrophil-driven inflammation to address the root cause of recurrent lung infections.
- FDA labeling highlights common adverse reactions such as upper respiratory infections, gingival and periodontal events, dermatologic reactions and hypertension, and includes precautions on live vaccines.
- Insmed has set a US list price of $88,000 per year for Brinsupri and expects net pricing to be 25–35% lower after rebates and discounts.
- Applications have been filed or accepted in Europe and the UK, with a planned Japan submission, and analysts forecast peak annual sales of $5–6 billion for the therapy.