Overview
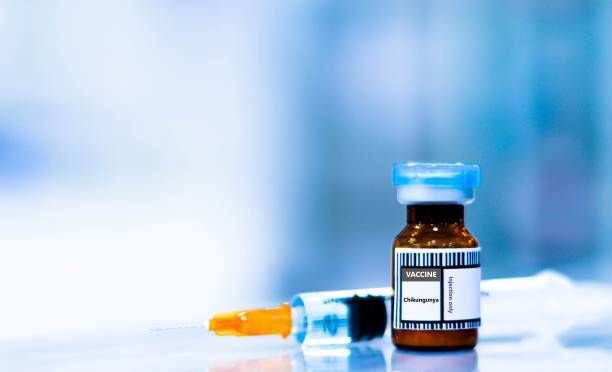
- The FDA and CDC have jointly recommended pausing the administration of Valneva’s IXCHIQ chikungunya vaccine for adults aged 65 and older due to reports of serious adverse events.
- The precaution follows six U.S. cases of heart or brain symptoms in older individuals with underlying conditions, reported shortly after vaccination, including one fatality.
- No causal link between the vaccine and adverse events has been confirmed, and investigations by U.S. and European regulators are ongoing.
- The vaccine remains authorized for individuals aged 18 to 60 in the U.S. and 12 to 64 in Europe, with over 40,000 doses administered globally.
- The U.S. government has advised travelers aged 60 and older to avoid the vaccine while safety reviews are conducted, and a second chikungunya vaccine is under consideration for broader use.