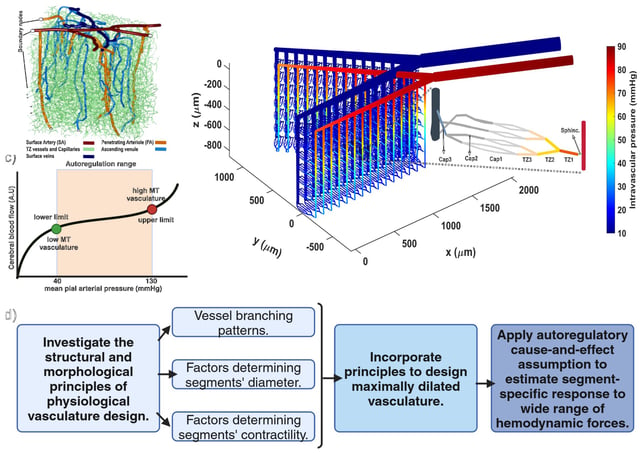
Overview
- The FAU team published an in-silico study in PLOS ONE that models each mouse brain microvessel segment as an adjustable valve to link vasodynamics with hemodynamics.
- Simulations reveal four distinct pressure-dependent flow regimes, including a stable ‘sweet spot’ and a high-pressure phase where vessels lose autoregulatory control.
- Transitional-zone vessels such as capillary sphincters and precapillary arterioles are shown to make the largest adjustments for baseline perfusion and functional hyperemia across cortical depths.
- Model outputs closely match experimental measurements in mice, validating the integrated approach to simulate microvascular flow and vessel response.
- Researchers say further refinement could extend the model to human brain and retinal imaging for non-invasive diagnostics, though clinical translation remains in development.