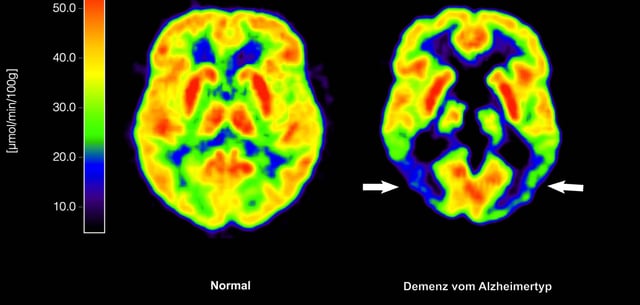
Overview
- The EU Commission has officially approved Lecanemab, the first antibody therapy targeting Alzheimer’s underlying pathology rather than just symptoms.
- The therapy is limited to patients with mild cognitive impairment or early dementia who meet specific genetic and clinical criteria, such as having one or no copies of the ApoE4 gene.
- Clinical trials showed a modest slowing of cognitive decline, with treated patients scoring slightly better on dementia scales over 18 months compared to controls.
- The therapy faces logistical and safety challenges, including risks of brain swelling and hemorrhages, and limited treatment capacity at specialized centers.
- High costs, estimated at over €23,000 annually per patient, and additional diagnostic expenses may further restrict access to the treatment.