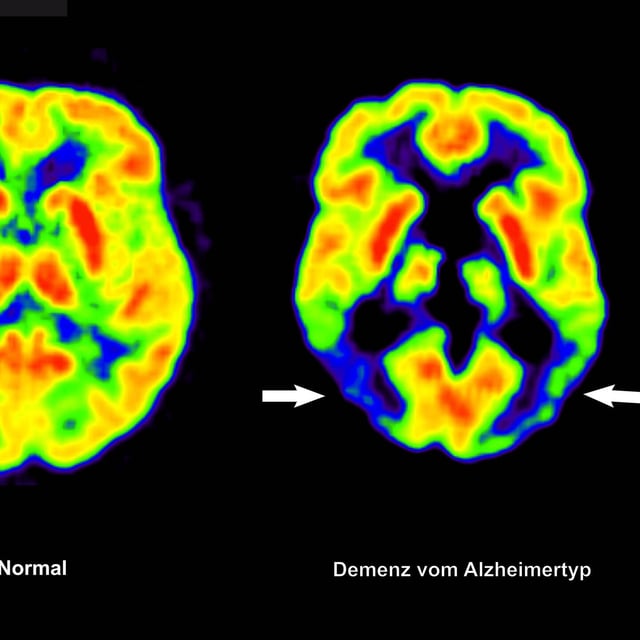
Overview
- The European Commission has approved Lecanemab, the first Alzheimer’s therapy in the EU aimed at modifying disease progression by targeting amyloid plaques.
- The drug is limited to early-stage Alzheimer’s patients with no or one copy of the ApoE4 gene to mitigate risks of brain swelling and bleeding.
- Clinical trials showed modest benefits, with treated patients experiencing a slower rate of cognitive decline, though the real-world impact remains uncertain.
- Preparations for rollout, including doctor training, infrastructure upgrades, and observational registers, are expected to delay availability by several months.
- Only a small subset of patients, estimated at around 20,000 in Germany, is eligible for treatment due to strict criteria and high costs.