Overview
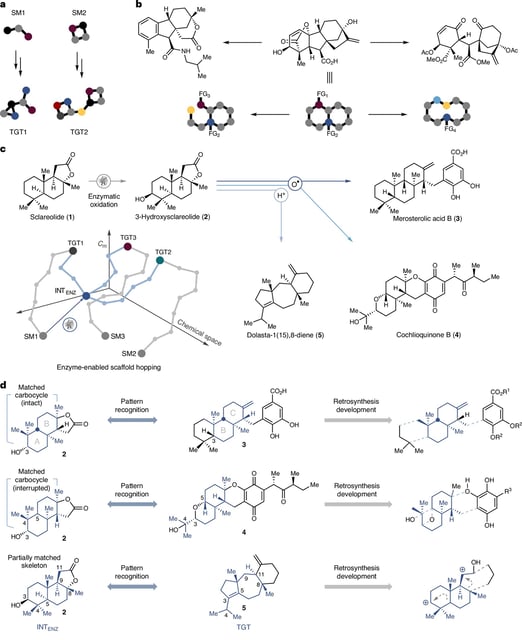
- Researchers engineered cytochrome enzymes to regioselectively oxidize the third carbon of commercially available sclareolide, producing a key alcohol intermediate.
- The team leveraged this biocatalytic hub to synthesize merosterolic acid B, cochlioquinone B, (+)-daucene and dolasta-1(15),8-diene from a single scaffold.
- This scaffold-hopping approach challenges conventional retrosynthetic design by proposing a shared entry point that branches into multiple synthetic pathways.
- By consolidating diverse terpenoid syntheses into one platform, the method promises to reduce steps, time and costs in natural product synthesis.
- Published June 16 in Nature Chemistry with support from the U.S. National Science Foundation and the Alfred P. Sloan Foundation, the strategy could accelerate medicinal chemistry research.