Overview
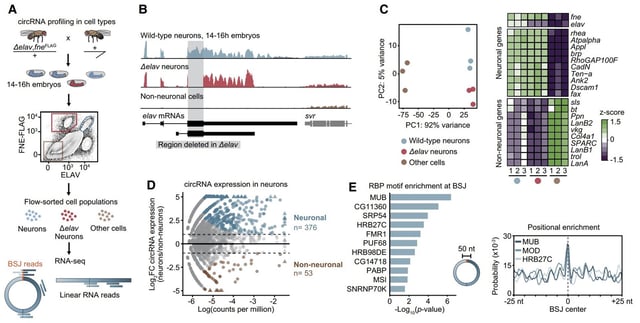
- Removing ELAV from Drosophila embryos reduces neuronal circular RNA levels by over 75%, while ectopic ELAV expression triggers new circRNA production in cells that normally produce few
- ELAV binds to pre-mRNA introns flanking backsplice junctions, slowing conventional splicing and bringing splice sites into proximity to drive circRNA biogenesis
- The closed-loop structure of circRNAs grants them extreme stability, making them long-lasting regulators of gene expression, synaptic function and neuronal development
- Proteins similar to ELAV are conserved from flies to humans, suggesting this splicing-switch mechanism underlies circRNA landscapes across animal nervous systems
- Targeting ELAV or its human analogs could enable modulation of circRNA levels, offering new strategies to study brain health and tackle neurodegenerative disorders