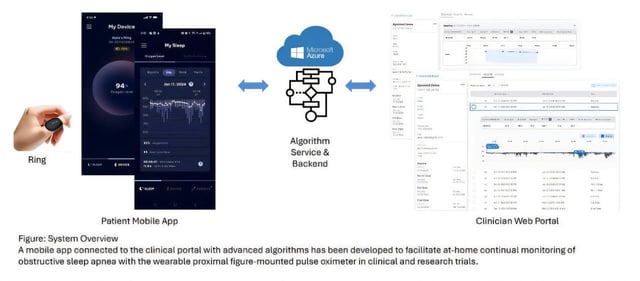
Overview
- The Connected Wearables platform integrates a ring pulse oximeter with a clinician dashboard to monitor sleep-related breathing disorders remotely.
- Clinical trials have collected over 130,000 hours of sleep data since July 2024, with 18,116 nightly recordings across 100 clinical sites.
- Trial results highlight an 85% patient adherence rate, with some patients voluntarily surpassing recommended usage periods.
- The device is FDA-cleared for medical use, while the connected software awaits regulatory approval for broader deployment beyond clinical trials.
- Researchers suggest multi-night data collection may provide more valuable insights than traditional single-night in-lab polysomnography.