Overview
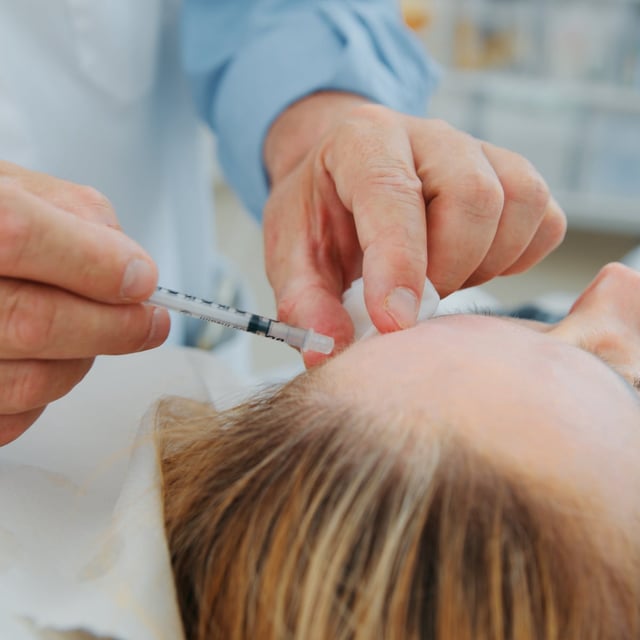
- At least six individuals in Tennessee and Illinois have been hospitalized due to botulism-like symptoms after receiving counterfeit Botox injections.
- The CDC and FDA are coordinating efforts to address the spread of these counterfeit products and have issued warnings to healthcare providers.
- Patients exhibited symptoms including blurred vision, drooping eyelids, and difficulty swallowing, indicative of botulism, a serious neurological condition.
- Health authorities stress the importance of receiving treatments from licensed professionals and in approved settings to avoid health risks.
- Investigations reveal that the counterfeit Botox was administered in non-medical settings such as homes and cosmetic spas.