Overview
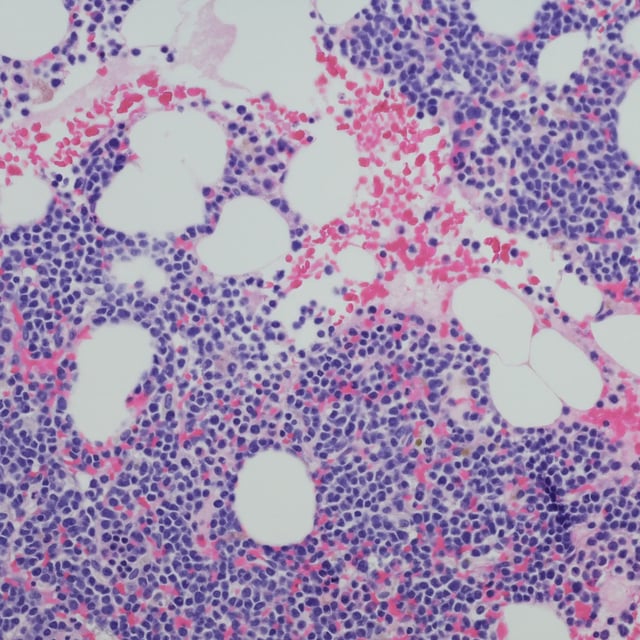
- A planned interim readout from the EXCALIBER-RRMM trial found iberdomide plus daratumumab and dexamethasone achieved significantly higher minimal residual disease–negativity rates than daratumumab, bortezomib and dexamethasone.
- On a recommendation from the Data Monitoring Committee, the study will continue without changes to assess the co-primary endpoint of progression-free survival as well as overall survival and safety.
- The two-stage, multicenter trial selected a 1.0 mg dose of iberdomide during stage 1 and then randomized approximately 664 patients in stage 2 to the investigational regimen versus the control arm.
- Investigators report a safety profile for the iberdomide combination that is generally consistent with prior studies.
- Bristol Myers plans to discuss the data with health authorities, and analysts view the MRD result as a positive sign while noting that PFS and upcoming head-to-head trials will define the drug’s role and commercial potential.