Overview
- IMA203, a TCR-engineered T cell therapy, demonstrated significant clinical responses in about half of the 40 patients with advanced solid tumors who had failed standard treatments.
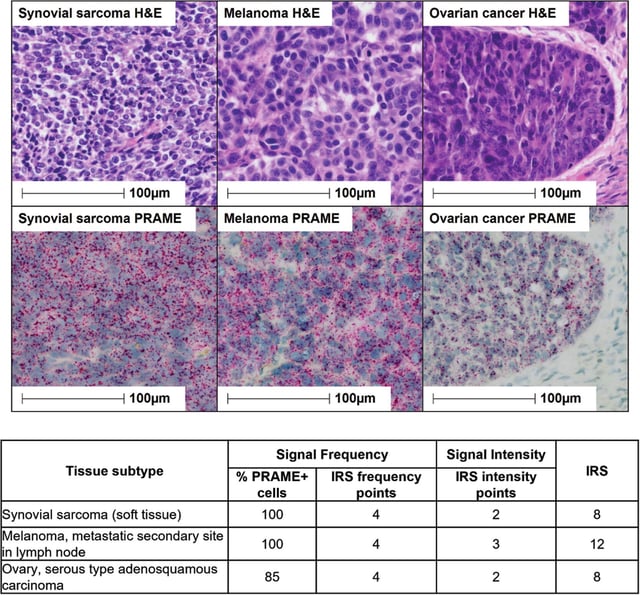
- The therapy targets the PRAME peptide, which is almost exclusively expressed by tumor cells, allowing precise cancer cell eradication while sparing healthy tissue.
- Patients experienced durable remissions, with some responses lasting over two years, marking a significant improvement over existing chemotherapy and immunotherapy options.
- The treatment exhibited a favorable safety profile, with side effects such as mild to moderate fever and skin rashes being temporary and manageable.
- Researchers at NCT/UCC Dresden are preparing to expand IMA203 into larger Phase 2/3 trials, initially focusing on melanoma patients resistant to conventional therapies.