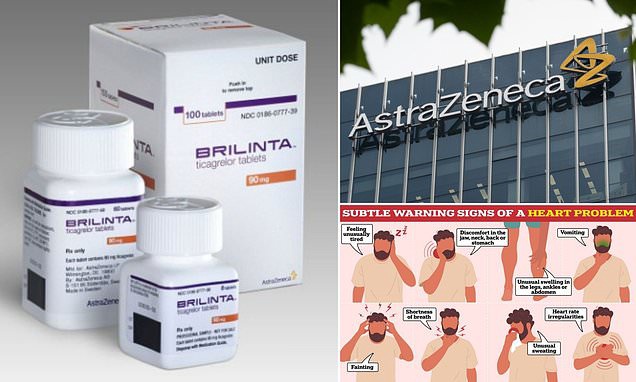
Overview
- The BMJ's latest investigation found two pivotal platelet studies underpinning ticagrelor's FDA approval misreported their primary endpoint results in Circulation.
- Over 60 of 282 readings from platelet machines were missing from the FDA datasets despite being central to efficacy analyses.
- Several trial investigators could not be reached or denied involvement, raising questions about author oversight and data integrity.
- Experts say unreported episodes of rebound platelet inhibition may leave patients vulnerable to dangerous clotting or bleeding.
- Regulators may face pressure to re-examine ticagrelor’s approval as generic versions are set to launch this year.