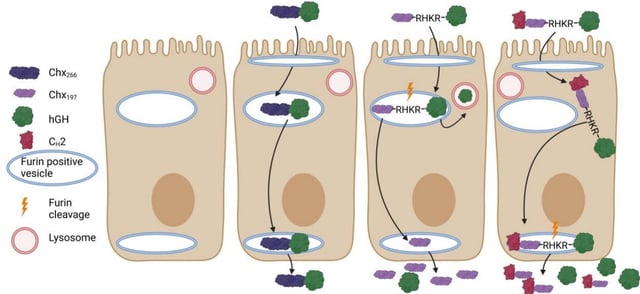
Overview
- The system attaches therapeutic proteins to a non-toxic carrier derived from cholera-associated bacteria to exploit a natural gut receptor pathway for transport.
- Rat studies showed consistent delivery of 5–10% of administered proteins into the bloodstream, meeting thresholds for commercial viability.
- Unlike prior approaches, this method preserves intestinal epithelium integrity and can accommodate diverse protein drugs.
- University of Bath scientists have teamed up with pharmaceutical firms to optimize dosages and formulations for clinical suitability.
- The researchers aim to launch initial human trials around 2027, pending further formulation refinement.