Overview
- Lecanemab (Leqembi) is now registered for adults with mild cognitive impairment or mild dementia due to Alzheimer’s disease, with the TGA stating eligibility is limited to APOE4 non‑carriers.
- Australia’s decision follows an administrative review after an initial refusal last year over safety and efficacy concerns in people carrying the APOE4 gene.
- A pivotal phase‑3 trial found about a 30% slowing of cognitive decline at 18 months, translating to roughly a six‑month delay in progression, with longer follow‑up suggesting growing benefit.
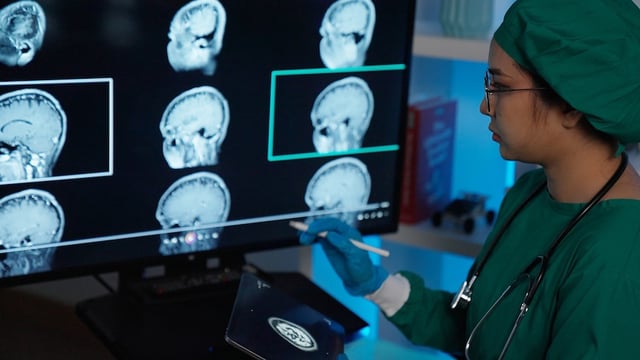
- The therapy can cause amyloid‑related imaging abnormalities, including brain swelling or small bleeds that are more common in APOE4 carriers, requiring frequent MRI monitoring and fortnightly IV infusions.
- Access and affordability remain major hurdles, with drug costs near A$39,974 a year and total care potentially approaching A$100,000 unless the medicine gains Pharmaceutical Benefits Scheme subsidy.