Overview
- Use is limited to adults with mild cognitive impairment or mild dementia due to Alzheimer’s who are APOE ε4 non-carriers or heterozygotes with confirmed amyloid pathology.
- The decision follows a February 2025 knockback and an Administrative Review Tribunal review requested by Eisai that led to an agreement with the regulator.
- Australia becomes the 50th country to register the therapy, which is administered as a one-hour intravenous infusion every two weeks to target amyloid.
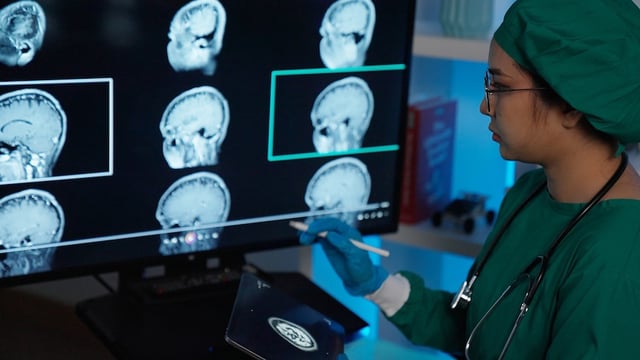
- Clinicians say the drug can slow decline in early disease, but monitoring for brain swelling or microbleeds is required, with MRI scans during treatment.
- The medicine costs about A$39,974 a year before infusion and imaging fees, and a Pharmaceutical Benefits Scheme listing has not been granted.