Overview
- The phase III ATOMIC trial enrolled 712 surgically resected stage III colon cancer patients with deficient DNA mismatch repair from 2017 to 2023.
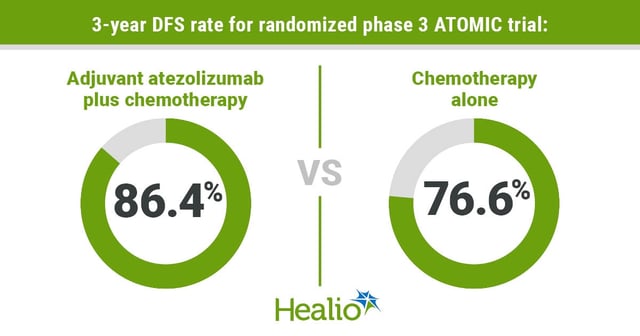
- Patients receiving atezolizumab plus standard FOLFOX chemotherapy achieved a 50% reduction in recurrence or death, raising three-year disease-free survival to 86.4% from 76.6%.
- Treatment combination was generally tolerable, with immune-related adverse events manageable alongside chemotherapy side effects.
- High mutational burden and active immune infiltration in dMMR tumors underpin the synergy between checkpoint inhibition and cytotoxic therapy.
- Investigators plan to submit the ASCO-presented findings to the NCCN to advocate for adopting the regimen as the new adjuvant standard.