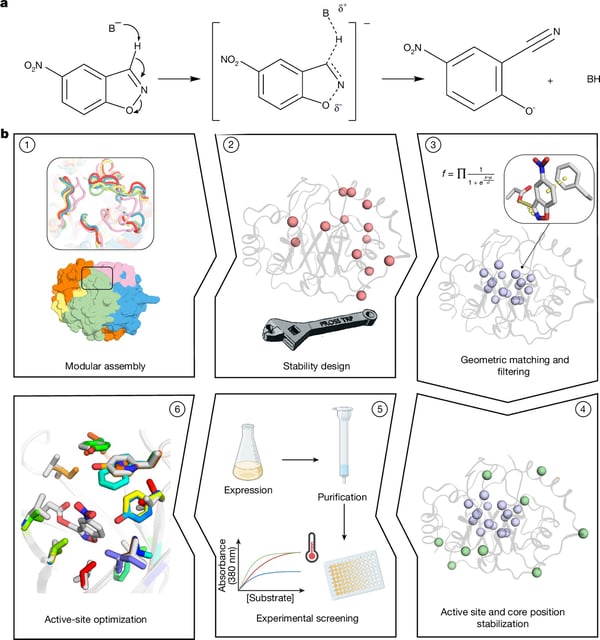
Overview
- The team used physics-based algorithms to assemble and optimize new protein backbones for the Kemp elimination, a reaction no natural enzyme catalyzes.
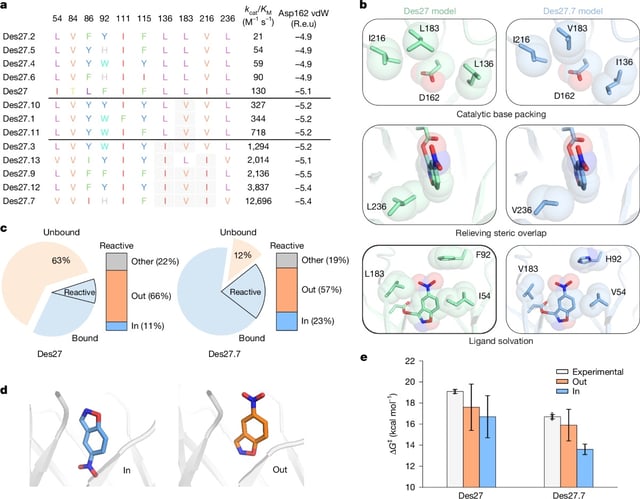
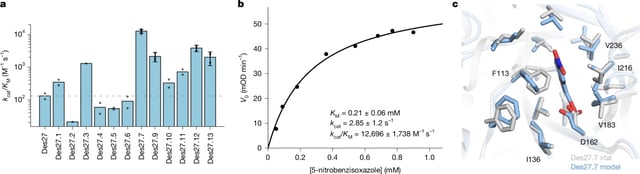
- Their most efficient variant diverged by over 140 amino acids from its closest natural relative and outperformed prior AI-designed enzymes by 100-fold.
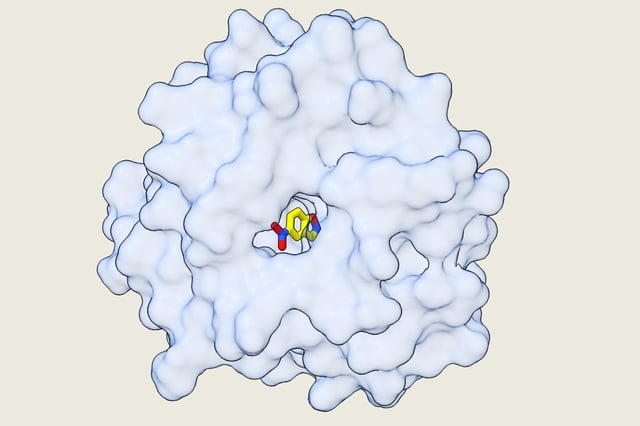
- Crystallography at near-2.1 Å resolution confirmed that computational models accurately captured active-site geometry and substrate positioning.
- Nano differential scanning fluorimetry demonstrated the synthetic enzymes maintain structural integrity under thermal stress, underscoring their robustness.
- This fully computational approach paves the way for rapid creation of tailored biocatalysts with applications in sustainable manufacturing, drug development and synthetic biology.