Overview
- Disposición 5842/2025 bans the use, distribution and commercialization of any falsified oral Ozempic presentation nationwide.
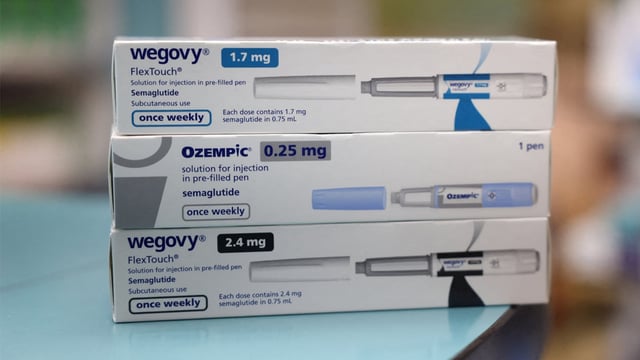
- ANMAT issued a public alert with side-by-side images of genuine and counterfeit products to aid identification.
- ANMAT referred the case to the Unidad Fiscal Especializada en Ciberdelincuencia and to Juzgado Criminal y Correccional N°18 with intervention by Fiscalía N°48.
- The counterfeit tablets were marketed on social media as “Ozempic Semaglutida Tablets USP, 25 mg, 60 tablets” falsely attributed to unregistered firms Pharma Argentina S.A. and MD Pharma.
- Consumers and health professionals are advised to verify traceability markings and obtain semaglutide injections only from authorized pharmacies.