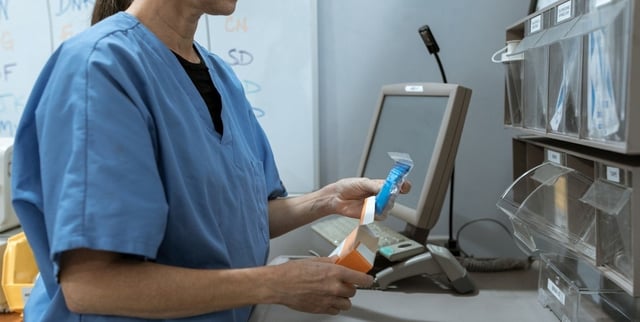
Overview
- Disposición 6223/2025, published in the Boletín Oficial, updates the Sistema Nacional de Trazabilidad to cover 495 active ingredients, including fentanyl and other estupefacientes and psychotropics.
- Oversight of substances such as morphine, oxycodone, methadone, ketamine, propofol, flunitrazepam and cannabidiol shifts from provincial paper vouchers to a centralized digital platform with real-time monitoring.
- Laboratories with already-registered products containing listed IFAs must comply within 45 business days, with updated REM requirements and INAME designated to implement the changes.
- The resolution sets technical criteria prioritizing high‑risk groups like oncologics, insulins, clotting factors, orphan drugs and implantable forms to curb falsification, illegal trade and fraud.
- The move follows WHO alerts and national probes into contaminated fentanyl, as ANMAT continues suspensions and market withdrawals affecting firms such as HLB Pharma, Laboratorios Ramallo and Rigecin Labs.