Overview
- Disposición 6223/2025, published Wednesday, expands mandatory tracking to estupefacientes and psychotropics with a published list of 495 active ingredients.
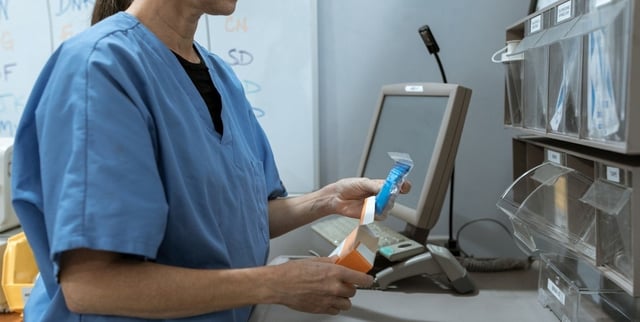
- Laboratories, importers and distributors have 45 business days to register and adapt their records to the national traceability platform.
- Fentanyl and other opioids transition from provincial paper-voucher oversight to centralized digital monitoring after the system went largely unchanged since 2016.
- Health officials say the upgrade enables real-time surveillance to detect irregularities, thefts or diversions to the black market and to trigger faster responses.
- Regulatory actions and probes continue, including suspensions and market withdrawals tied to HLB/Ramallo and Rigecin following a WHO alert on contaminated fentanyl lots.