Overview
- ANMAT's Disposición 5842/2025 prohibits the sale, use and distribution nationwide of any product labeled “Ozempíc Semaglutida Tablets USP.”
- The move followed a Novo Nordisk complaint and a sample confirming the tablets promoted on social media are counterfeit and that no oral Ozempic exists globally.
- Regulators determined the firms named on the packaging—Pharma Argentina S.A. and MD Pharma—are not registered or authorized to make medicines.
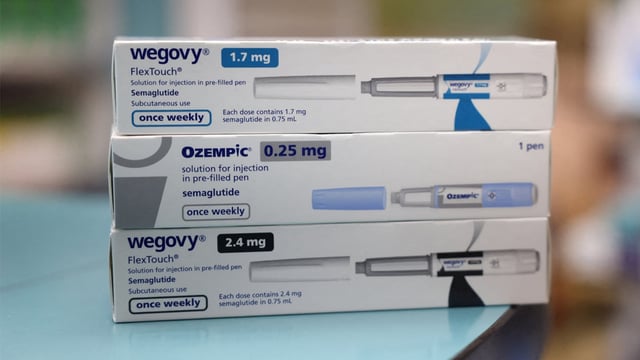
- The real Ozempic available in Argentina is a prescription injectable with unit traceability, whereas the falsified tablets pose health risks because their composition is unknown.
- ANMAT published a public alert with comparative images and referred the case to UFECI, which forwarded it to Juzgado Criminal y Correccional Nº 18 with Fiscalía Nº 48.